The basic building blocks of proteins. A peptide bond is a type of covalent bond between the carboxyl group of one amino acid and the amino group of another amino acid.

Ch 2 Chemistry Of Biology Flashcards Quizlet
VOCABULARY SHEET CH13 Chemical bonding Chemical bonding the joining of atoms to form new substances Chemical bond a force of attraction that holds two atoms together Valence electrons the electrons in the outermost energy level of an atom Ionic bond the force of attraction between oppositely charged ions Ions charged particles that form during chemical changes.
. Chemical Bonds Vocabulary Chemical Bond The force that holds atoms or ions together as a unit. Start studying Pearson Interactive Science. Insoluble- A substance that does not dissolve in water.
A compound is a substance made of atoms of two or more elements bonded together in a certain ratio. Dissolve- A substance that mixes into water to make an aqueous solution. Conductivity- A property that has to deal with whether a substance will conduct electricity.
An ionic bond forms when one atom gives up one or more electrons to another atom. A group of atoms held together by covalent bonds and having no overall charge. Amino acids are joined by peptide bonds.
Chemical Formula Notation that shows what elements a. Building Vocabulary Fill in the blank to complete each statement. Tendancy of ionic compound to break and not bend.
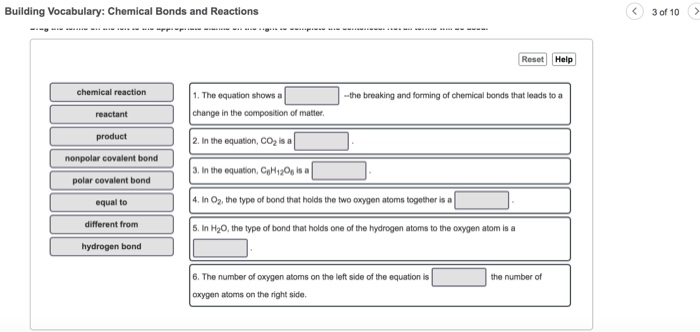
In the equation CH120 isa reactant product nonpolar covalent bond polar covalent bond equal to different from hydrogen bond. A chemical bond is a force of attraction between. Learn reactions vocabulary chemical bonds with free interactive flashcards.
Conductivity is a property that describes how well a substance transmits electricity heat or sound. Ion an atom or groups of atoms that has a positive or negative charge. A charged chemical species ion composed of two or more atoms covalently bonded or of a metal complex that can be considered to be acting as a single unit.
The force of attraction that holds two atoms together is called an _____ 8. How are the two terms related. The Potential energy that is stored in the chemical bonds that hold chemical compounds together.
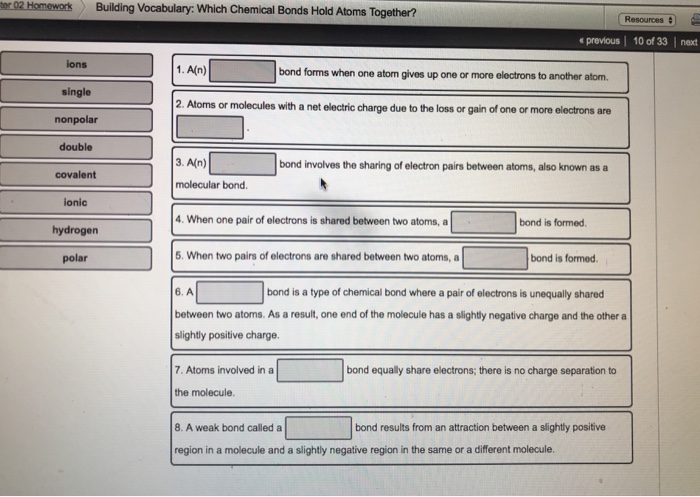
2 Atoms or molecules with a net electric charge due to the loss or gain of one or more electrons are _____. A chemical bond in which one atom loses an electron to form a positive ion and the other atom gains to electron to form a negative ion. Ionic bonds involve the transfer of one or more electrons.
The bond creates stability between the atoms. A force that holds atoms together to form molecules. A combination of substances in which individual substances retain their own properties.
In O2 the type of bond that holds the two oxygen atoms together is a 5. When one pair of electrons. Building a Better Vocabulary- Some words that express Annoyance and Disgust Gadfly n.
The attractive force that holds atoms or ions together. A covalent bond is formed when atoms share valence electrons. In the year 1927 valence bond theory was formulated which argued essentially that a chemical bond forms when two valence electrons in their respective atomic orbitals work or function to hold two nuclei together by virtue of system energy lowering effectsIn 1931 building on this theory chemist Linus Pauling published what some consider one of the most important papers.
Cation An ion with a positive charge. Main Types of Chemical Bonds. By sharing electrons or 2.
An covalent bond involves the sharing of electron pairs between atoms also known as a molecular bond. An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. The equation shows a the breaking and forming of chemical bonds that leads to a change in the composition of matter 2.
A Chemical Bond is an attraction between atoms that holds them together. Log in Sign up. By transferring electrons and creating two oppositely charged atoms Covalent bonds involve the sharing of one or more electrons between atoms.
A group of atoms bonded together representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction. Atoms with large differences in electronegativity transfer electrons to form ions. The two main types of bonds formed between atoms are ionic bonds and covalent bonds.
Start studying chemical bonds and reactions vocabulary. The force of attraction that holds two atoms together as a result of the rearrangement of electrons between them. 5272018 Chapter 2 316 ANSWER.
Soluble- A substance that dissolves in water. Group of atoms held together by a chemical bond. To Dissolv e something is to disperse a substance homogeneously into another.
Correct In chemical reactions atoms gain release or share electrons. A mixture in which one or more substances are distributed. Chemical bonds are forces that hold atoms together to make compounds or molecules.
Bond formed between metal atoms where the electrons are shared amongst them. Biology - Chapter 7 Vocabulary. Covalent Bonding is a type of bonding in which one or more pairs of valence electrons are shared between the atoms.
Atoms or molecules with a net electric charge due to the loss or gain of one or more electrons are ions. Chemical bonds include covalent polar covalent and ionic bonds. There are two main ways that atoms can bond together to form molecules.
Amino acids themselves are made of atoms joined together by covalent bonds. A large complex polymer make of CHON and sometimes S with subunits of amino acids. Ionic Bond The force that holds cations and anions together.
An atoms _____ are those electrons that are in the highest energy level. In the equation CO2 is a 3. Chemical Bonds and Compounds Vocabulary.
Have students scan the text on this page to find the definition of each term. Force of attractions that holds atoms together as a result of the rearranging of the electrons between the atoms. A protein that changes the rate of a chemical reaction.
An enzyme that speeds up a chemical reaction. Which Chemical Bonds Hold Atoms Together. Learn vocabulary terms and more with flashcards games and other study tools.
Choose from 500 different sets of reactions vocabulary chemical bonds flashcards on Quizlet. The primary structure of a protein consists of amino acids chained to each other. Call on students to read aloud the definitions.
Building Vocabulary Help students distinguish between the closely related terms chemical bond and chemical reaction. The atoms do not always share the electrons equally so a. 1 An _____ bond forms when one atom gives up one or more electrons to another atom.
Chemical bond- What chemists call the attraction that holds atoms together. Atoms in a molecule can be. A molecule is a group of atoms held together by one or more covalent bonds.
Learn vocabulary terms and more with flashcards games and other study tools. Anion An ion with a negative charge. An atom or group of atoms the gains or loses electrons and has an electrical charge.
3 An _____ bond involves the sharing of electron pairs between atoms also known as. Each element is represented in the periodic table by an _____. Atoms with relatively similar electronegativities share electrons between them and are connected by covalent bonds.
Structure formed in an ionic bond where the ions form a repeating three dimensional pattern. The bonds that connect amino acids. A covalent bond is a chemical bond that forms when valence electrons are shared between atoms.
Solved Building Vocabulary Chemical Bonds And Reactions 3 Chegg Com

Ch 2 Chemistry Of Biology Flashcards Quizlet

Solved Er 02 Homework Building Vocabulary Which Chemical Chegg Com

Solved Concept Hw Locabulary Which Chemical Bonds Hold Chegg Com
0 Comments